Excell’s Guide To Metal Finishing’s
Share this article:

The Different Types of Metal Finishing's
Each type of metal finish has its own unique application process and benefits, and like metal spinning, each of these is a craft of its own. Excell specialises in metal spinning, and so most of the metal finishing’s are outsourced.
This blog is a guide and combination of the metal finishes that we provide in-house, as well as just some of the hundreds of metal finishes available. It will cover the finishing’s, the processes, and methods for doing them, and the metals that they are best suited to work on.
Ultimately, the overarching theme is that these methods are mostly designed and used for their protective qualities, however, many of the following methods provide some very visually appealing results.
Excell’s Metal Finishes – Scotch-Brite, Bright Polish, and Brush Finishing:
Scotch-Brite:
This is our standard finish – Scotch-Brite is a semi-dull finishing process that produces a uniform grain and unidirectional texture. This removes any spinning lines made in the spinning process and polishes the metal.
Scotch-Brite Pad:
This is our standard finish – Scotch-Brite is a semi-dull finishing process that produces a uniform grain and unidirectional texture. This removes any spinning lines made in the spinning process and polishes the metal.
Scotch-Brite Flap Discs:
This is an Aluminium Oxide and Scotch-Brite abrasive disc that can be used as an extension on power tools. This is commonly for light deburring, cleaning, polishing when finishing and preparing metals.
We used these discs in the grades P40 and P60. The P40 is useful when removing a heavy stock from a surface, and P60 is to remove a slight amount of stock, as well as refine the finish.
These processes can be done on the following metals:
Aluminium
Stainless Steel
Mild Steel
Copper
Brass
Bright:
This is not quite a mirror finish but is very bright and reflective of light. There are different grades of smoothness of this polish. This can be done in-house at Excell but comes at an extra cost. This is done using a separate polishing machine that uses ‘polishing mops’ to polish the metal.
This can be done on the following metals:
Aluminium
Stainless Steel
Copper
Brass
This finish is best on Stainless Steel as it gets a very bright finish.
Brush Finishing:
Brush Finishing is separated into two categories: Grit Finish and RA Finish. Brush Finishing is essentially where an abrasive material such as emery cloth or sandpaper, is held onto the spinning as it is spun, to get an even abrased finish. In the case of Stainless Steel, it can be bought pre-brushed, however, if it is completed post-spin, the finish is much more even and of a higher quality.
Grit finish:
A grit finish is a standard brush finish. For this process, the customer requests the grade finish they require. The sandpaper we use at Excell is Emery, which comes in a wide range of grades, and the customer can select any grit grade between 40 and 240, in increments of 20.
RA finish:
An RA Finish is a certain high spec grade of brush finishing, that is usually requested for medical-grade jobs. This is requestable by specifying an RA Code. This is the specification that the company and use of the product will require. This is done using a special machine that will regulate how much pressure and stability is provided while the metal is being abrased.
Lubricants and Finishing Processes:
Scotch-Brite is always used at the end of the spinning process. This is used as a cleaning process to ensure that each spin is free of lubricant and spinning lines, this is usually the stage that spins are left at and is the standard finish that we send spins out at unless the customer requires a different finish. The lubricant used must be removed at the end of the spinning process, this can be done by using the Scotch-Brite pad, dipped in paraffin. Paraffin helps break down any of the, most commonly, oil-based lubricants, and allows the general Scotch-Brite polishing and the cleaning to be completed in one step.
Certain lubricants can be used for different metals. The following lubricants are those that Excell uses most commonly. These are as follows:
Tube Draw:
Tube Draw is an oil-based and lubricant that is the consistency of syrup and is often used when spinning Stainless Steel.
Tallow:
Tallow is essentially lard. This is commonly used on Aluminium, Copper and Brass spins.
Grippo:
This is an in-house made wax product. This lubricant is an all-rounder lubricant that can be used on any metal.
Natural Soap:
Meaning the base form of natural, unscented, and untreated soap.
Outsourced/Specialist Metal Finishes
The list of possibilities on how to finish a metal are endless, and some go into a very specialist realm of manufacturing. Some are for visual purposes, and some for practical. As these finishes are trades and services of their own, these are services that we can outsource for our customers.
Paint – Powder Coating
Powder Coat Paint is a dry finishing process. The powder paint is made up of finely ground particles of resin and pigments, as well as additives for specific functions. These can be, for example; gloss or hardness.
Before the application, the part must be thoroughly cleaned to remove all oils. The powder is then applied using a spray gun, which is fitted with an electrode that provides an electrostatic charge to the powder as it leaves the nib of the gun. This charge means that the powder is attracted to a grounded part, i.e the part being coated, and this holds it there until it is melted and fused into a uniform coating in the curing oven.
Paint – Wet Paint Coating
Wet paint coating is the traditional metal finishing process.
This involves the application of a paint, in liquid form, to a metal surface. This can be done in several ways, for example, using a spray, pump, or pressurized vessel.
To begin the paint application, the metal part needs to be cleaned thoroughly to remove any oils, and then the paint can be applied. The paint is sprayed on in 15-20 micrometre increments. This is continued until the part is fully coated, and the paint has reached the desired thickness.
Benefits of Wet Paint:
- Metal finished help protect the metal from any damage or corrosion.
- Out of the paint options of powder and wet, wet will only be used when powder is not the better option.
- An advantage of wet paint over powder is that it allows for thinner coats to be applied.
- Because curing of the paint occurs at room temperature, this method can be used on metals that are heat sensitive.
- If over time, the product becomes scratched or damaged, touch-ups using wet paint coating are easily achieved.
Customisations:
Wet paint is available in a larger range of colours than powder coating, and there is a higher degree of precision when creating and choosing custom colours.
Price vs Durability:
Wet paint is the better option when coating a smaller batch, however powder coating provides a more durable finish.
Ingredients:
Wet: Resins, additives, pigments, solvent.
Powder: Resins, additives, pigments. – Powder coating does not contain solvents as it is applied as a powder.
Passivating:
The passivation process usually involves the formation of an outer protective layer on the metal that adds extra corrosion protection, as well as adding an extra sheen.
The reaction that occurs during the passivation process is technically a form of corrosion that takes place as the metal reacts with the environment. However, this “corrosion” layer that forms, will reduce and almost eliminate any further corrosion.
The metals that can be passivated are Stainless Steel, Aluminium and Copper, and the process for each is different.
The one common step is the cleaning process. Before the passivation process can begin, the material must be cleaned thoroughly, to remove any oils on the surface that could interfere with the passivation.
Stainless-Steel:
Background on STainless-steel's structure:
Every stainless steel has a natural corrosion resistance built-in, however, this does not mean that it is immune to rusting over a prolonged time and extreme conditions.
The metal part is then submerged into an acid bath of the specific chemical and temperature requirements for the grade of passivation, then rinsed and dried.
The chemistry behind the process:
The acid passivating bath removes the exogenous iron that replaces it with a passive oxide layer that will prevent any further rust from forming. This layer is less than 0.0000001 of an inch.
Passivation of stainless steel will be classified by industry standards and codes.
3 different acid chemical mixtures can be used in the stainless-steel passivation process.
- Nitric Acid
- Nitric Acid with Sodium Dichromate
- Citric Acid
The chemical used in the passivation bath depends on the grade classification criteria.
Copper:
The passivation of copper is slightly different to the other metals, as there is no way to truly alter copper using chemicals.
There are two ways to ‘passivate’ copper:
Exposure to the natural elements:
This is the easiest way to passivate copper, however, it does not allow for time-sensitive projects. This involves simply leaving them outside between four and eight weeks. Over this time a dull green-blue film will start to form. This will act as a preventative for any further corrosion. This film that forms is also referred to as a patina.
Chemical Bath:
This process is similar to the traditional passivation process, however, the chemicals involved are different. To do this, add together 3 kilograms of ammonium sulphate, a third of a cup of copper sulphate, an eighth of a cup of ammonia and 24 litres of water. (Highett Metal)
Unlike traditional passivation, this does not involve dipping the part into the solution, instead, it needs to be sprayed onto the copper. A total of 6 layers should be applied to the copper, and it must be left to dry between each application.
Once the last application has been applied, it must be left to completely dry for six hours, and over that time the colour of the patina will begin to develop and deepen in colour.
Ultimately to protect the copper from corrosion, it must be allowed to corrode initially.
As a metal, copper can simply be left to form self-made protection against corrosion, but if the colour is something that is specifically sought after, the latter step can be approached.
Aluminium:
Background on Aluminium's structure and properties:
When pure aluminium is exposed to oxygen, it undergoes a natural process of oxidation. This is where a thin layer of aluminium oxide forms on the surface. While this layer works for some aluminium alloys, others need passivation as extra protection.
There are two ways to passivate aluminium:
Chromate conversion coating:
These coatings can vary in thickness from 0.00001 to 0.00004 inches. They do not have a uniform shape or structure and when hydrated with water, have a jelly-like consistency. This acts as a barrier between aluminium and the threat of corrosion.
Anodizing:
Anodizing, as well as a way to passivate aluminium, some other metals can be anodized, and the process is explained below.
Anodizing:
Anodizing itself is a recognised standalone and frequency requested process of metal finishing, but it falls into the category of aluminium passivation.
This process is more similar to the process of Stainless-Steel Passivation. Anodizing is an electrolytic chemical passivation process that thickens the pre-existing oxide layer of the metal to one that is more durable, resistant to corrosion and that has an Adonic oxide finish. The difference between the naturally occurring layer from exposure to oxygen is that the new layer created is a more hydrated composition of aluminium oxide, that is more resistant.
Most commonly used/requested is coloured anodizing, where the oxide surface of the metal can be dyed. The new layer that is created is a more hydrated composition of aluminium oxide, which is more resistant than the already present layer.
The anodizing process is done by immersing the aluminium into an electrolyte bath of acid, where an electric current is then passed through the medium.
The purpose of anodizing is to increase the thickness of the natural oxide layer on the metal’s surface. This increases the metal’s resistance to corrosion and wear.
The most suitable material to anodize is Aluminium, however, it can be used on other metals such as Magnesium, Titanium, or any other copper-alloy metal.
Hot Dip Galvanizing
The Hot Dip Galvanization process has been used since the 1700s. This process can stand the test of time and gives maintenance-free protection against corrosion. Since the creation of this process, the steps and technicalities have changed and developed as technologies have become more advanced.
Process:
This process involves dipping steel or iron into a bath of zinc that has been melted. This is a coat of zinc-iron alloy and zinc metal and acts as corrosion resistance.
Steel is a type of iron alloy, which means that all types and forms of steel contain iron.
This is what makes the galvanization process possible. As the steel/iron is submerged in the zinc bath, a metallurgical reaction between the zinc and the iron takes place.
The reaction that takes place is known as a diffusion process. This means that the coating it creates is uniform in thickness over the full surface of the part.
The entire process is completed by hanging the part from a specially designed dipping rock and is moved from place to place for each step.
Surface Preparation - Caustic Cleaning:
This is the first stage of surface preparation before the galvanizing can be done. The surface of the metal must be thoroughly cleaned and free of any oils and oxides.
Caustic cleaning is a method of cleaning that corrodes the metal using a chemical reaction. For this stage, the steel or iron is cleaned in a bath of degreasing solution. A solution commonly used are usually alkaline solutions. Once the caustic degreasing cleaning is complete; the part is rinsed with water.
Pickling:
The ‘pickling’ solution that the part is next dipped into, is either hydrochloric or sulfuric acid.
This solution reacts with the steel and iron to remove all oxides and any mill scale.
Once again, the part is rinsed with water.
Flux Solution:
Flux’s main purpose is to prepare the steel or iron for ‘soldering’. In this case, the soldering can refer to the galvanized metal fusing to the part being galvanized. This also acts as a protective coating that will prevent the oxides from reforming as it is moved to be galvanized. A common solution for fluxing is zinc chloride and ammonium chloride.
There are technically 2 separate flux solutions, the one mentioned previously, and a layer of flux that sits on top of the zinc layer in the galvanizing bath.
This layer ultimately ensures that in the time between the flux and the zinc bath, that there are no contaminants that may have reformed or attached.
Before the galvanization step, the part must first be left to dry.
Galvanization - Zinc Bath:
The zinc that is in the bath has been specified to ASTM B6. This is a standard specification document for zinc. This document specifies whether the zinc is any one of the three types of zinc, all of which are at least 98% pure zinc.
This zinc mixture can sometimes contain other metals to promote certain qualities.
The steel that is dipped will stay in the bath until it has reached the temperature of the bath., which is 438°-460°C. The dip lasts less than 10 minutes, but it depends on how long the part takes to reach the temperature for exactly how long it stays in the bath.
Post-Treatment:
It is optional to have post-treatment, but these are just to enhance the galvanized coating. Common post-treatment is quenching. This is where the hardness of the galvanization is ‘locked in’ This entails the metal being rapidly cooled after being taken out of the zinc bath – which hardens the coating.
The quench tank will contain mostly water but can also optionally have chemicals that will protect the galvanized layer by adding a layer of passivation. This will be for protection while transporting or storage.
As a final step, the part is cleaned, and any dripped spikes of iron are removed by sanding and grinding.
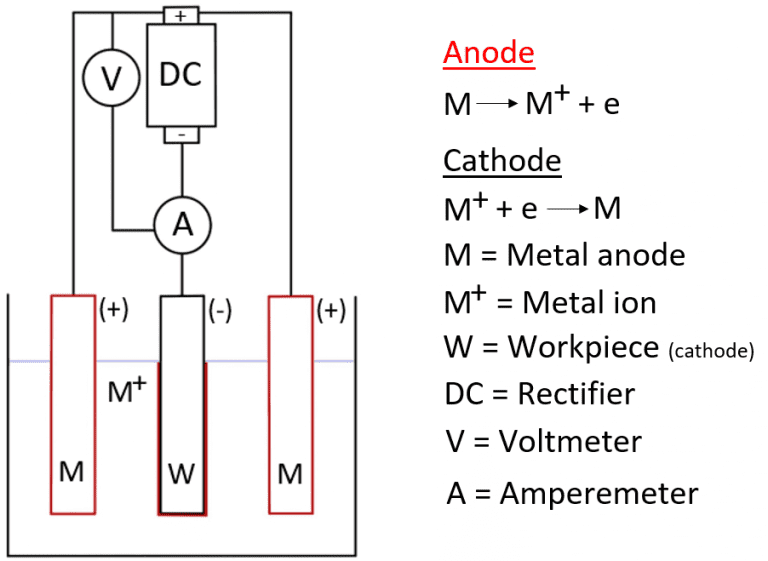
Electroplating:
Electroplating is the process of plating a part with a thin layer of the desired plaiting material by using an electrolytic procedure. This is achieved by passing a current through the electrodes as they are submerged in the electrolyte bath. The cathode is the part that is to be plated, this process is called electrodeposition.
There are several varying materials that can be used; however, the electroplating procedure stays the same, it is the anode and the electrolytic bath that will interchange depending on the desired result.
Each plating material has its own benefits and qualities for best practice. This also means that the elements of the anode and electrolyte bath will need to be altered accordingly.
The Core of Electroplating:
The Science Behind It:
Plating is done by using an electrolytic bath, however, there is a lot of science behind the process, which is called Electrolysis.
For the electroplating process, two electrodes are submerged into a bath of suitable electrolyte solution for the desired plating results, where a current is passed between the two electrodes, one positive and one negative.
The negative is the cathode, which is the part to be plated; the positive is the anode, which will most commonly be a rod of suitable alloy or metal that will enable the plating process to work effectively.
As the current is passed through the electrodes to the electrolytic bath, the atoms within the electrolyte bath will move towards and attach to the cathode.
Plating and Electrolyte Mediums:
There are several alternatives when it comes to plating, however, this blog will highlight some suitable and relevant options for the industry of manufacturing.
Copper:
The electrolyte solution required to electroplate with copper can be salt water or a copper sulphate solution. Using copper sulphate instead of salt water solution because this means that the elemental copper within the surface can be harnessed for the plating, opposed to using a copper anode.
As well as this, when using a walt water solution, it is possible that the salts can break down into chlorine gas and be released. Chlorine gas can be very harmful, and so the process will need to be completed under suitable lab ventilation when using salt water as an electrolyte solution.
While a salt solution may be more versatile or cheaper option, copper sulphate could be an overall better option when considering the pros and cons of both.
The metals that can be Copper plated are Silver, Aluminium and Gold. Any iron-based metals such as steel, will need to be plated with nickel as copper does not easily adhere to a surface containing iron.
As copper can be a very expensive material, copper plating could be a more affordable option if the visual element of copper is a feature that you are particularly seeking.
Chrome:
The metals that can be Chrome-plated are Stainless-Steel, Mild Steel, Copper, and Brass.
There are two types of Chrome Plating:
Hard Chrome – also referred to as Engineered or Industrial Chrome.
Decorative Chrome
Hard chrome can not only be useful in increasing corrosion resistance, but also in repairing any damaged and worn parts by helping build them back up to size. Where they can be reshaped to the required and appropriate size. To rebuild the shape of a worn part, the layer will need to be very thick and so depending on the requirements and desired uses, hard chrome can vary in thickness. The process of hard chrome is low friction and has high strength and durability.
On the other hand, decorative chrome is a much finer layer as it is purely for the visual aesthetic, and to add colour to the part. Due to this it is slightly more limited in its variety but has a role in every industry. Some of its common uses are for car parts, tools, kitchen utensils and musical instrument hardware. While it is mostly used for the visual element, it does also provide a very light corrosion protection layer. However, it is not anywhere near as durable as hard chroming, simply because the layer is much thinner and will wear faster.
Zinc:
Zinc plating is also known as Galvanizing, as discussed in the previous section.
Electropolishing:
Electroplating is also known as electrochemical polishing, anodic polishing, or electrolytic polishing.
This process in its simplicity, is the removing of a thin surface layer on a metal part.
This is typically performed on Stainless Steel, but some of the other compatible metals are Aluminium and Brass.
The scientific process of electropolishing is like passivation, but the two are in fact very different. Both electropolishing and passivation are non-mechanical and chemical bath processes. The two can almost be thought of as reverses of each other, while passivation adds a small layer, electropolishing takes a small layer away.
Scientific Process of Electropolishing:
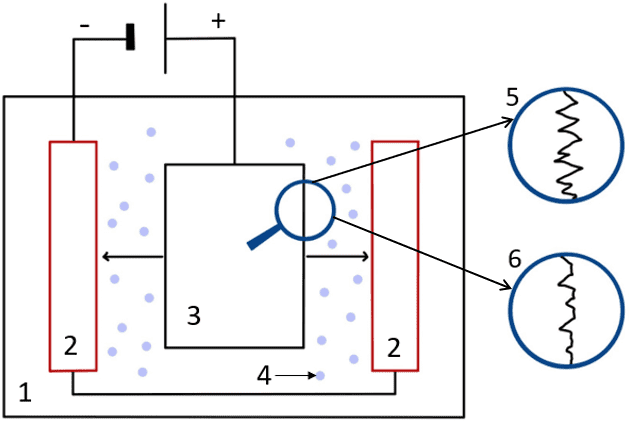
- Electrolyte
- Cathode
- Workpiece (Anode)
- Particles moving towards the cathode
- Surface before polishing
- Surface after polishing
The electrolytic solution that is commonly used in electropolishing, typically consists of a high-viscosity mixture of sulfuric acid and phosphoric acid. The electrical current travelling through the electrolyte causes the metal ions on the surface of the part to oxidize, which then dissolves in the electrolyte.
This process involves a natural phenomenon known as anodic levelling. This occurs during the electropolishing process when areas with higher burrs, peaks of areas with more roughness, erode faster than the smoother areas. This is because these irregular areas attract a greater current density, and so erode first and faster.
Just Some of The Professions And Industries That Use Electropolishing:
Medical: Keeps tools clean and functioning well.
Pharmaceutical, food and beverages: electropolishing materials stay clean and are easily sterilizable.
Aerospace and Automotive: the use of electropolishing materials in manufacturing reduces friction, enhances performance, and prolongs the lifetime of the parts
Conclusion and Summary:
After discussing the above metal finishes, it is evident that choosing the correct finish for your metal is crucial. To receive the desired attributes such as hardness, water resistance and corrosion resistance, the correct finish must be chosen for the metal of your part. Many of these processes are universal on the metals they can be used on, but a select few are only compatible on one or two metal types, due to the structure of the metals.
As well as this, there are some metals that you would not use a specific finish on, and another may be a better choice. For example, it is extremely uncommon to use a Powder coating finish on copper. This is not because it physically cannot be done, but because copper is a very expensive metal, and is commonly chosen for its rich colour, and covering it with a colour would leave the choice of metal null and void. Instead, a cheaper metal with the same properties could be used and then coloured. This will give the same result but will be much more cost effective.
Summary of Metal Finish Compatibility:
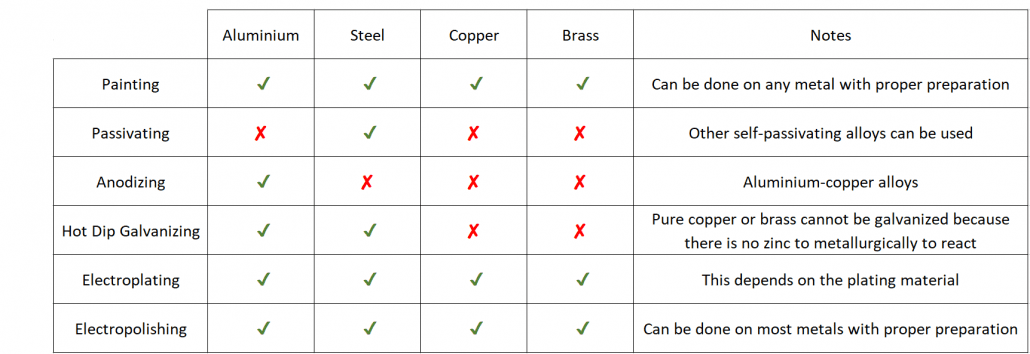
Table of Contents
ExcellMetalSpinning
Related Articles

Boosting British Manufacturing: A £360 Million Investment
Boosting British Manufacturing: A £360 Million Investment Facebook Twitter LinkedIn WhatsApp Email It’s been declared ahead of the Budget that Chancellor Jeremy Hunt will announce
Design Considerations for Metal Spinning Success: Part One
There are many design considerations businesses should considers to get the most out of their metal spinning supply. Read Part One here

The Importance of Innovation in UK Manufacturing
Manufacturing plays a pivotal role within the UK, shaping society and innovating like no other. It is the most productive sector in the UK

